Cell BiotechProbiotics Specialists
Probiotics for Life
Cell Biotech specializes in developing innovative probiotic solutions to meet rapidly growing consumer demands in the global market.
Since its establishment by microbiologists, Cell Biotech has been striving to support healthier lives for customers through probiotics.
Our Research & Development Department sets us apart from other brands in all areas, from strain selection to finished product, which we export to forty countries around the world.
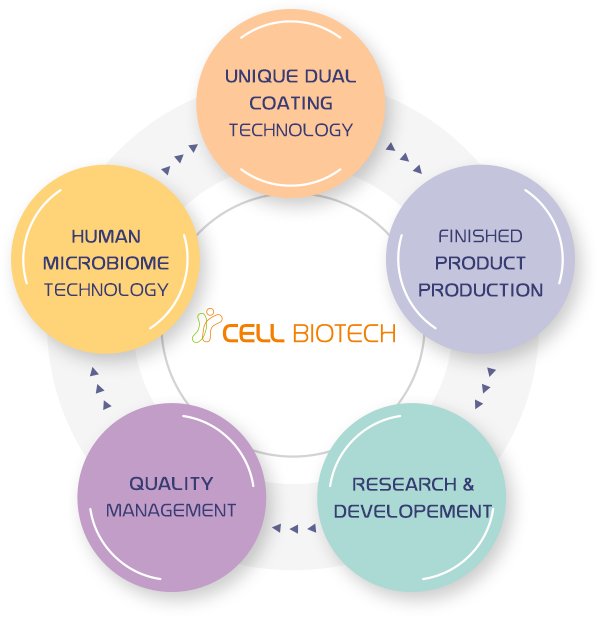

TOTAL SOLUTION PROVIDEROne-Stop Solution Optimized Cutting-Edge Facilities Used Exclusively for Probiotics
We provide reliable probiotic products through our one-stop solution that covers strain development, production, distribution, quality control, and customer service in accordance with global standards.



01Strain Isolation & Research
Cell Biotech strains are isolated from healthy human and traditional Korean fermented foods. All Cell Biotech strains are safely managed and studied for various functional efficacies.
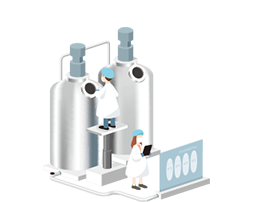
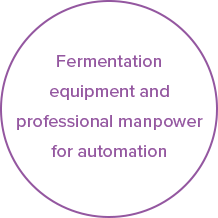
02Fully Automated Lactic Acid Bacteria Fermentation
Our fully automated fermentation process allows Cell Biotech to produce various probiotics products under a set of high-quality standards. To ensure the optimal state of living bacteria, we have optimized the fermentation conditions for each strain and established mass production facilities.Read more
To ensure optimal state of living bacteria, we have optimized the fermentation conditions for each strain and established mass production facilities through continuous technology innovations as a means to secure global-scale fermentation facilities.


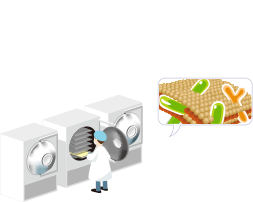
03Dual Coating and Freeze-Drying
To increase the survival rate of probiotics, all Cell Biotech products are protected by a dual-coating matrix, which consists of protein and polysaccharides, providing enhanced moisture control for all raw materials.
04Strain Cocktailing Process
The raw material is mixed using strain cocktail technology, which ensures the complete blocking of harmful external material.
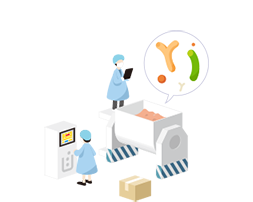
05Finished Product Production
We produce products in various dosage forms so that customers can select the products which best suit them.Read more
 Production facilities specialize in probiotics for global exports.
Production facilities specialize in probiotics for global exports.
Customized probiotics are available in various dosage forms such as powder, tablets, capsules, and oil drops.
06Quality Inspection
With a world-class quality control system, Cell Biotech conducts quality inspections at each and every stage, from raw material inspections to product delivery. Cell Biotech has established a quality control system to meet different criteria of various countries, and this allow us to export to over forty countries including the United States, Europe, and the Middle East.Read more



With a world-class quality control system, Cell Biotech conducts quality inspections at each and every stage, from raw material inspections to product delivery. Cell Biotech has established a quality control system to meet different criteria of various countries, and this allow us to export to over forty countries including the United States, Europe, and the Middle East.
Compliance with Global Safety Standards
We have implemented globally approved quality inspection to ensure the reliability as well as safety of our products and have obtained various certifications such as Halal, Kosher and ISO, which allow us to export to more than forty countries all over the world.




* Halal and Kosher certifications are only valid for the Singapore product range and specific raw material.
07Shipping and Customer Service
All of our products are delivered in a safe and timely manner through our own logistics system. We are ready to respond to customer requests in order to provide the best possible service.
R&D CenterOur Laboratory
A team of 51 microbiological specialists carry out scientific research in a professional probiotics research facility. They primarily study medical probiotics, bacteriocin and the natural antibacterial substances. They are expanding the range of applications not only to health supplements but also biocosmetics and biomedicine.
Learn more
- Strain Management
- Clinical / Non-Clinical Research
- Microbiome Research
- Microbial Technology Research
- Colon Cancer Treatment Research
- Bio-Cosmetic Research
History
2019
- Business agreement with K-BIO for colon cancer treatment R&D
- The 4th plant for colon cancer treatment constructed
2018
- LACTOClear Launched (patent for lactic acid bacteria drug delivery system and recombinant lactic acid bacteria for anticancer treatment registered proved effect of colon cancer treatment, GLP Biotoxtech)
2017
- Selected as World Class Product of Korea
2015
- Selected as one of ‘World Class 300’ (Small & Medium Business Administration)
2013
- The 3rd plant (finished product production) constructed
2010
- LAB brand DUOLAC launched ‘Technology Innovation of the Year’ (Frost & Sullivan) received
2008
- Patent for dual-cation LAB (DUOLAC) in Europe registered
2006
- Cell Biotech International (Denmark) established
2004
- Patent for dual coating LAB (Korea, Japan) registered
- HALAL certification attained (Korea Muslim Federation)
2002
- FDA safety approved (USA) - HACCP certification attained (SWISS, SGS) - Listed on KOSDAQ
- HACCP certification attained (SWISS, SGS)
- Listed on KOSDAQ
2000
- ISO-9001 certification attained (UK, BM TRADA)
- 12 GBMO certification attained (Korea Food & Drug Administration)
1996
- The 2nd production plant constructed (finished product manufacturing – GMP facilities)
- Cell Biotech International established
1995
- Cell Biotech established
- The 1st production plant constructed (fermentation plant)
- Bio-Cellular Engineering Laboratory established


